Effective January 25, 2015, NIH has revised its definition of “clinical trial.” The revision is designed to make the distinction between clinical trials and clinical research studies clearer and to enhance the precision of the information NIH collects, tracks, and reports on clinical trials.
Clinical Trial Definition: A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. (Notice of Revised NIH Definition of “Clinical Trial”)
- A health-related biomedical or behavioral outcome is defined as the pre-specified goal(s) or condition(s) that reflect the effect of one or more interventions on human subjects’ biomedical or behavioral status or quality of life. Examples include:
- Positive or negative changes to physiological or biological parameters (e.g., improvement of lung capacity, gene expression)
- Positive or negative changes to psychological or neurodevelopmental parameters (e.g., mood management intervention for smokers; reading comprehension and /or information retention)
- Positive or negative changes to disease processes
- Positive or negative changes to health-related behaviors
- Positive or negative changes to quality of life
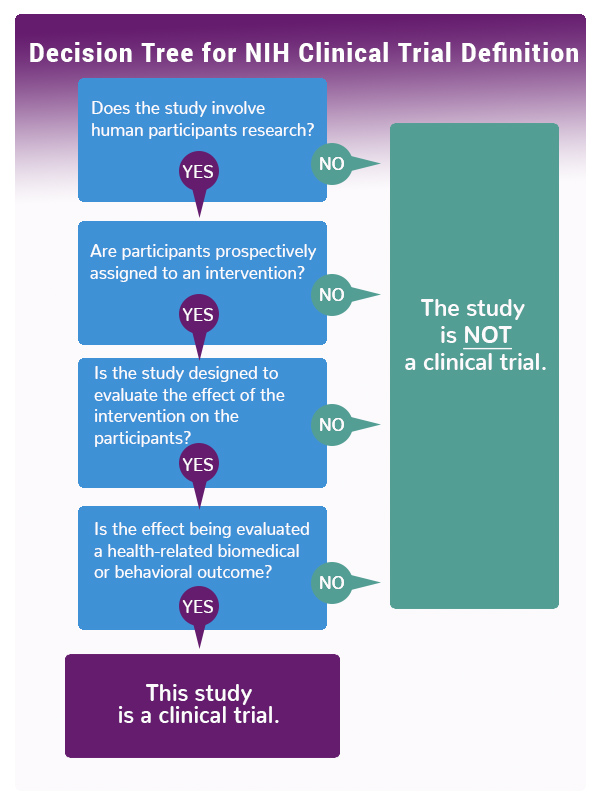
The decision tree below can be used to help determine if your research meets the NIH definition of a clinical trial.
Need more help?
Please use these additional resources provided by NIH to help determine if your research study meets the definition of a clinical trial:
- Clinical Trial Definition FAQ’s (Additional definition clarifications and helpful information)
- Clinical Trial Definition Case Studies (Simplified case studies that apply the four key decision tree questions to determine whether NIH would consider the research study to be a clinical trial)